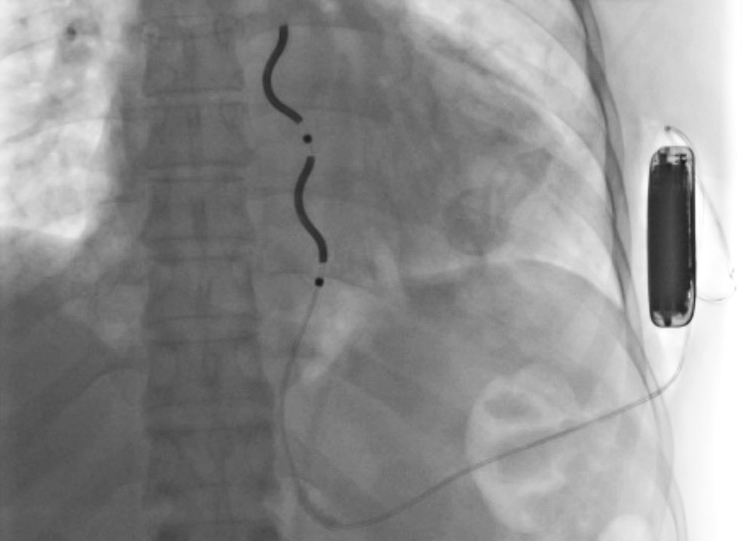
Implantable cardioverter defibrillators (ICDs) successfully detect and stop arrhythmias like ventricular tachycardia and ventricular fibrillation. Now, a newly released ICD that avoids some of the challenges of traditional ICDs is being introduced at a select few medical centers in the U.S., including UVA Health. The lead on the device — the extravascular implantable cardioverter-defibrillator (EV-ICD) — is placed directly on top of the heart in the retrosternal space rather than within the blood vessels.
What’s New About EV-ICDs
With traditional transvenous ICDs, lead wires are threaded through blood vessels and implanted directly into the heart. However, this can lead to complications, like vascular injury. If there is an infection (such as from sepsis), malfunction, or if an upgrade is needed, you must perform an invasive and sometimes risky lead extraction procedure to remove the device.
Pamela Mason, MD, a professor of cardiovascular medicine at UVA Health, recently led the team that implanted the first EV-ICD in Virginia. “This breakthrough technology is an important advancement in treating abnormal heart rhythms,” says Mason.
The device, which was approved last fall by the Food and Drug Administration (FDA), is indicated for patients who have experienced — or are at significant risk of developing — life-threatening ventricular tachyarrhythmias. According to the trial results from the study published in the New England Journal of Medicine, the device showed a 98.7% effectiveness at delivering defibrillation therapy.
Location Helps Avoid Complications
In the EV-ICD, the defibrillator lead is implanted beneath the sternum in the anterior mediastinal space. “This location prevents vascular complications that traditional transvenous ICD leads can cause, which include infection, vascular occlusions, and tricuspid regurgitation,” says Mason.
Like the subcutaneous ICD (S-ICD), the EV-ICD’s generator is implanted below the left armpit (in the left mid-axillary region). But with S-ICD, the lead is above the sternum.
Mason points out another advantage: “The leads in the new device are solidly constructed, unlike the hollow leads in transvenous defibrillators. Therefore, we think they are going to be much more robust and durable,” she says.
The EV-ICD’s generator is roughly half the size of, and can last up to twice as long as, the S-ICD’s generator. That’s because when it delivers a shock, the EV-ICD is closer to the heart and doesn’t have bone between it and the heart. The shock outputs can be lower, helping the battery last longer. The device’s manufacturer projects a longevity of nearly 12 years.
Limited Pacemaker Capabilities
Unlike the S-ICD, which lacks pacemaker capabilities, the EV-ICD’s pacing feature monitors the heart for significant pauses and responds by providing temporary bradycardia pacing support. The device also delivers post-shock pacing after defibrillation or cardioversion therapy. “Providing limited pacing therapy to patients for acute issues, including anti-tachycardia pacing, is an important feature of the EV-ICD device,” says Mason.
However, she cautions that patients with significant and ongoing pacing requirements will likely need to be fitted with a transvenous device, rather than the EV-ICD. “The device is also contraindicated for patients who have had a prior sternotomy,” Mason adds.
Importance of Multidisciplinary Team
To successfully implement an EV-ICD program, medical centers should have a multidisciplinary team in place. In fact, the FDA requires the presence of surgeons for the first 5 cases performed. This is because the leads go behind the sternum into the anterior mediastinal space.
“Our multidisciplinary team — cardiologists, surgeons, electrophysiologists, and anesthesiologists — went through a formal training with [the manufacturer] and we created a credentialing document regarding the EV-ICD,” says Mason. “We believe that our strong track record and relationships among our team was a key factor in being chosen to be one of a handful of centers nationally to initiate implementation of the new device.”
Improved Patient Comfort
When patients receive a transvenous defibrillator, their arm mobility is typically restricted for the first several weeks after the procedure. For those who do physical work, like lifting their arms above their shoulders, this restriction is particularly frustrating.
With the EV-ICD, however, mobility restrictions generally are much shorter — typically just a week or so.
Patients with EV-ICDs will also notice cosmetic improvements. Transvenous ICDs in the shoulder can be seen when patients wear swimsuits or tank tops. But, with its location on the side, the EV-ICD is more easily hidden.