Two groundbreaking brain tumor medications UVA Cancer Center researchers helped investigate are currently awaiting FDA approval. If they receive it, the drugs could make a significant difference in the way the tumors are treated — and in the lives of patients.
The first drug, vorasidenib, would become the first FDA-approved targeted therapy for isocitrate dehydrogenase (IDH)-mutated grade 2 glioma. This type of low-grade glioma typically becomes refractory to treatment and is, eventually, fatal. In a clinical trial, vorasidenib slowed tumor growth significantly and extended the average length of time before the tumor started growing from 11.1 months to more than 27 months.
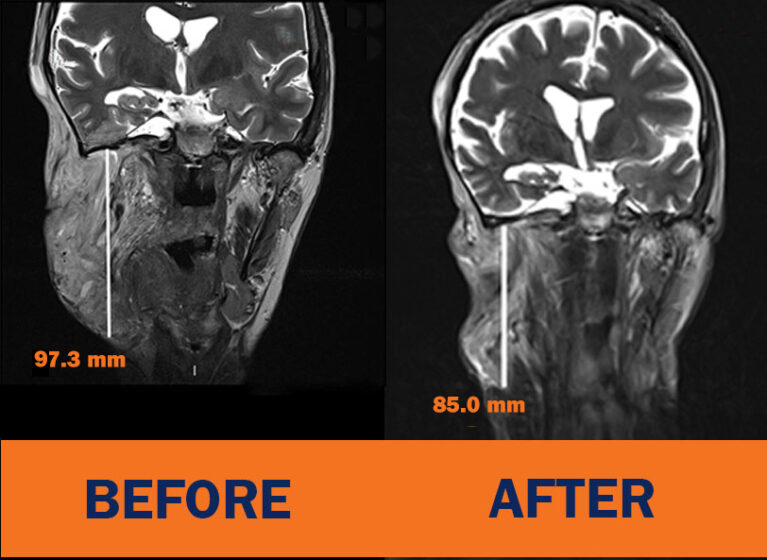
The second drug, mirdametinib, would become the first FDA-approved medication for adults with neurofibromatosis type 1-associated plexiform neurofibromas (NF1-PN). A recent trial showed “impressive” results in both children and adults with NF1-PN, says David Schiff, MD, co-director of UVA Cancer Center’s neuro-oncology center. Roughly 40% of study participants — both pediatric and adult — experienced tumor shrinkage, as well as less pain and improved quality of life.
Longer, Better Lives With Vorasidenib
IDH-mutated grade 2 glioma affects around 2,500 people in the United States each year. Many of these patients are younger, with 40 as the median age of diagnosis. They often suffer from tumor-related epilepsy and cognitive issues.
Although the tumors are slow growing, the average life expectancy for people who develop them is about 10 to 15 years. The standard treatment of radiation therapy and alkylating chemotherapy agents tends to include toxic side effects, like lower blood counts, gastrointestinal distress, fertility effects, and difficulties with short-term memory and concentration.
Because of the limited treatment options, many doctors advise their patients to “watch and wait” before prescribing therapy, to get a sense of the tumor’s growth rate.
The INDIGO clinical trial randomized patients who received the experimental drug with patients who received a placebo. The patients who received vorasidenib — an investigational inhibitor of IDH-mutant enzymes — lived longer. They also didn’t need the alkylating chemotherapy agents or radiation as quickly as patients who received the placebo.
“For many patients, I think the results of this trial will make this drug a more enticing approach than taking a watch-and-wait approach,” Schiff says. “The idea of being able to delay the time until people have to get these potentially more toxic therapies is very appealing.”
The FDA is expected to grant approval of vorasidenib for use in patients with IDH-mutated grade 2 glioma by Aug. 20.
In a New England Journal of Medicine editorial about the drug’s potential, Schiff wrote that it could “put a nail in the coffin of the watch-and-wait approach” to treating the tumor. He also points to other opportunities that could come from the drug’s approval: “Could this be useful in patients with grade 3 gliomas, which aren’t that different from grade 2 gliomas? We now understand we can use molecular targeting against IDH-mutant gliomas to exploit their biologic differences.”
Significant Tumor Shrinkage With Mirdametinib
Neurofibromatosis type 1 (NF1) often causes plexiform neurofibromas (PNs) to develop. These benign tumors can grow aggressively on the covering of nerves and lead to pain, disfigurement, and other health concerns in patients.
In 2000, the FDA approved selumetinib for pediatric patients with NF1 who have inoperable, symptomatic PNs. The kinase inhibitor was designed to block the overactive MAPK pathway in these patients. Last year, drug manufacturer SpringWorks Therapeutics, Inc., announced the results of the phase 2 ReNeu clinical trial of mirdametinib. This investigational drug is designed to inhibit MEK1 and MEK2, which occupy key positions in the MAPK pathway, this shrinks tumors in both pediatric and adult patients.
“Again, the results were pretty impressive,” Schiff, who participated in the trial, says. He notes that 52% of pediatric patients and 41% of adult patients experienced tumor shrinkage when taking mirdametinib. Both pediatric and adult patients also reported significant improvements in pain, quality of life, and physical function. Also, Schiff says, the results seem durable and the drug itself well-tolerated.
SpringWorks is now applying to the FDA for approval of mirdametinib. Additional data from the ReNeu trial are being presented at the American Society of Clinical Oncology (ASCO) meeting this June.
“Assuming mirdametinib is approved, we’ll have — for the first time — an FDA-approved drug for adults with plexiform neurofibromas,” Schiff says. “Previously, they really had no effective treatment options.”
Schiff adds that future implications for the drug’s development include studying whether it could be useful in combination with other therapies, as well as whether it could be useful in patients with NF1 who have types of tumors other than PNs.
