UVA Health System is now offering patients with a ventricular arrhythmia, such as ventricular tachycardia or ventricular fibrillation, a less invasive alternative to the standard implantable cardioverter defibrillator (ICD). According to UVA cardiologist Andrew Darby, MD, the subcutaneous ICD or S-ICD helps restore a regular heart rhythm by delivering a higher energy shock through the chest wall rather than through an intracardiac lead.
“The S-ICD generates a higher energy shock than standard ICDs,” explains Darby. “Most transvenous ICDs are capable of generating a 40-joule shock, whereas the S-ICD generates 80 joules. Generating higher energy ensures that enough energy reaches the heart to stop the arrhythmia.”
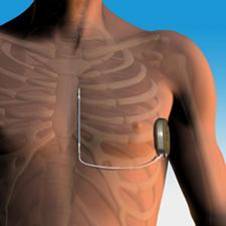
While the S-ICD device consists of similar components – a “can” with a generator and sensing software, as well as wiring that delivers the shock – the placement of it is very different from the standard ICD. This difference is most notable in the wires, which are placed under the skin and not into the vasculature. This is important because it reduces the risk for bloodstream infections, according to cardiologist Pamela Mason, MD. Without that risk, the S-ICD may be a better option for a wider subset of patients.
“This device is well suited particularly for very young patients because they are more prone to vascular complications over the long term, as well as those who are on dialysis or who are prone to infections,” says Mason.
Fully approved by the U.S. Food and Drug Administration, the S-ICD is currently being offered at only a handful of centers in the region. At UVA, patients benefit from the expertise of highly trained physicians and electrophysiology team, as well as the continuity of care provided by a well-established heart program.
“At UVA, we have outstanding pacemaker and ICD clinics for follow-up of these devices,” says Darby. “These device clinics are not only at UVA, but also at a number of other locations throughout central Virginia, including Orange, Fishersville, Zion Crossroads, Culpepper and Charlottesville, which allows us to provide comprehensive service to patients throughout the region.”
Subcutaneous Implantable Cardioverter Defibrillator
Overview
The S-ICD is an implantable defibrillator that is placed under the skin and does not connect directly to the heart. It offers defibrillating capacity (it can shock the heart), but does not have pacing capabilities like standard transvenous ICDs.
Candidates
Candidates for an S-ICD include:
- Patients with a prior ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation)
- Patients at increased risk for sudden cardiac death (those with a prior heart attack and reduced ventricular function; those with reduced heart function from primary heart muscle disorders; and those with certain genetic conditions that place them at risk for ventricular arrhythmias)
- Younger patients who may require an ICD for long periods
- Patients at risk for bloodstream infections
- Patients who have had past problems with transvenous ICDs
Exclusions
Because the device lacks pacing capability, the primary exclusion is the need for a pacemaker. This may include patients with symptomatic bradycardia or those with ventricular arrhythmias that are well tolerated and can be terminated by pacing the ventricles rapidly.
Device Placement
Because the wires of the S-ICD are not placed into the vasculature, fluoroscopy guidance is not required during the procedure, limiting radiation exposure. Instead, anatomic landmarks are used to determine where the device should be placed. The ICD generator, or “can”, is placed in the left midaxillary line (which is located just down from the middle of the axilla, or armpit) and the lead is tunneled under the skin and placed about 1 cm lateral to the sternum (breastbone).
Follow-Up
The patient will be seen every three months for monitoring.
Lifespan
Like the transvenous ICD, the S-ICD has a lifespan of approximately seven years.
Read more about UVA Heart Rhythm Center.