At a Glance
- UVA Advanced Cardiac Valve Center is one of the few sites in the U.S. offering the full spectrum of treatment options for repairing and replacing all cardiac valves.
- Hospitals performing a higher volume of transcatheter aortic valve replacement (TAVR) procedures have better outcomes.
- Since 2009, UVA has performed nearly 400 TAVR procedures.
- UVA providers have helped centers across the state establish new TAVR programs.
- As one of eight accredited core members of the Cardiothoracic Surgical Trials Network, UVA is involved in high-profile trials that directly impact the way cardiovascular disease is treated worldwide.
Key Considerations for Valve Referrals
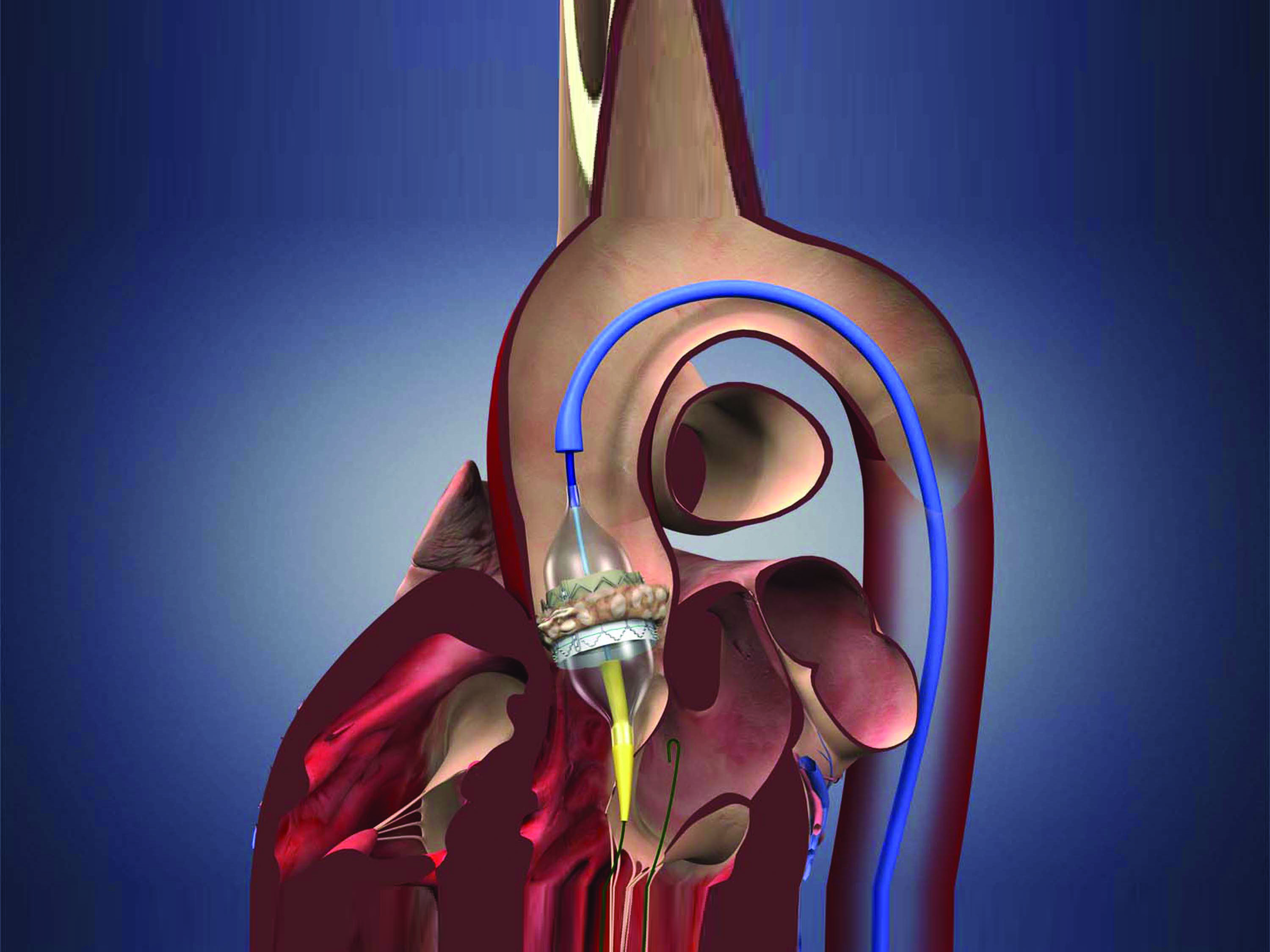
The rapid evolution in treatments for heart valve disease over the past decade has provided novel and viable options for high-risk and inoperable patients with life-threatening heart valve conditions. Yet, as new devices and surgical techniques become more widely available, accessibility is just part of the equation when determining where best to refer a patient for valve procedures.
Higher Volumes, Better Outcomes
At the American College of Cardiology Annual Scientific Session held in April, researchers reported that hospitals that perform a high volume of transcatheter aortic valve replacement (TAVR) procedures have better outcomes. This report is based on an observational study of more than 40,000 patients on the Transcatheter Valve Therapy (TVT) registry. The study evaluated how many TAVR procedures were performed at nearly 400 U.S. hospitals and how often patients experienced death, vascular complications, bleeding or stroke following the procedure. The result: a statistically significant association between higher volume and reduced mortality.
“This recent study further confirms that a center’s depth of experience translates to better outcomes for patients,” says Scott Lim, MD, medical director of the UVA Advanced Cardiac Valve Center, one of only a few sites in the U.S. offering the full spectrum of treatment options for repairing and replacing all cardiac valves, including surgical, minimally invasive and transcatheter approaches. The UVA team has been performing TAVR since it was first introduced in 2009, as one of 26 U.S. sites selected to participate in the initial PARTNER (Placement of Aortic Transcatheter valve) trial. Since that time, UVA has completed nearly 400 TAVR procedures. That’s significantly above the national average.
“We were truly at the forefront of this treatment,” says Gorav Ailawadi, MD, surgical director of the UVA Advanced Cardiac Valve Center. “Since that first study, we have been involved in more than four additional clinical trials for the newest TAVR devices.”
A Measure of Quality
Intuitively, the TAVR observational study findings make sense: practice makes perfect. With greater volumes come greater skill and greater exposure to a variety of cases. Yet, at UVA, the measure of a quality valve program goes beyond the number of patients treated to include attributes more difficult to quantify. These attributes not only affect patient outcomes, but also the advancement of valve therapies worldwide.
Collaboration
The valve team at UVA includes not only highly experienced cardiac surgeons and interventional cardiologists, but also GI specialists, radiologists, pulmonologists, nephrologists, ICU staff and others dedicated to serving this patient population. “We rely on the expertise of many across the entire spectrum of care,” says Ailawadi. “We have weekly meetings, and all are involved in discussing available options to ensure the best outcomes for our patients.”
As an example, Ailawadi describes the role of nephrologists who may provide insight into how to minimize the risk for kidney injury during surgery, as well as radiologists specially trained in reading CT scans who help guide the team to make the procedure safer for the patient. In addition, because patients who require valve surgery often are older, there is an added benefit and sense of security that comes from having a variety of highly trained specialists on standby to treat any peripheral conditions that may be diagnosed.
Versatility
As you’re probably aware, not all patients with valve disease qualify for minimally invasive procedures like TAVR or MitraClip® (percutaneous mitral valve repair used to treat severe degenerative mitral regurgitation). At UVA, these patients have access to the full range of surgical treatments as well, including minimally invasive and standard approaches, performed by leading specialists in the field. In 2015, UVA was one of only 27 hospitals to achieve the highest, three-star ratingfor aortic valve replacement (AVR) from the Society of Thoracic Surgeons.
“Just as it’s been shown that high-volume centers have better outcomes with TAVR, the same is true of surgical valve repair and replacement,” says Ailawadi. “We not only do a high volume of cases, we do those that are more complex and may not be not done elsewhere. We have become a destination for many patients as well as other physicians learning to do these procedures.”
Read more about surgical outcomes for valve procedures performed at UVA in our Clinical Activity Report for Cardiothoracic and Vascular Surgery.
Knowledge Sharing
Because of their experience and favorable outcomes, the UVA Advanced Cardiac Valve team has been a resource for both device companies seeking to develop newer, more effective treatments and other centers across the country looking to expand their valve disease programs.
UVA was the first international training center for MitraClip®, educating cardiologists and surgeons worldwide on the nuances of placing the device. UVA providers have also been sharing their knowledge on TAVR since it became FDA-approved.
“We do whatever we can to support centers that want to adopt this latest technology,” says Lim. “Our team has helped establish new TAVR programs across the state through training and ongoing consultation.
Device companies turn to UVA for insider perspective on how to improve their products. “We have a say in designing new trials — where technology is going, the type of patients eligible, how to make devices better and easier to use — for technology that is approved,” says Ailawadi. “We’re also asked to participate in feasibility trials for new devices like those used in transcathether mitral valve replacement and tricuspid valve repair.”
Discovery
UVA has a long history of participation in valve studies and continues to push for safer approaches and ways to bring the latest technology to more patients. As one of eight accredited core members of the Cardiothoracic Surgical Trials Network (CTSN), sponsored by the National Institutes of Health/National Heart Lung and Blood Institute, UVA researchers are involved in high-profile clinical trials that directly impact the way we treat cardiovascular disease nationwide.
“Because of our involvement in clinical trials, we provide our patients with access to the next generation of valve technology and we’re able to offer valve replacement to more patients with aortic valve disease,” says Lim. “Other centers in our region are limited to treating only those who are high risk or inoperable,” says Lim.
Some of the valve studies occurring at UVA include:
- Concomitant Tricuspid Repair During Mitral Valve Surgery
Patients with mitral valve disease often have concomitant tricuspid valve regurgitation (TR). Through the CTSN, UVA is embarking on an international landmark study to investigate the safety and effectiveness of concomitant tricuspid repair even in patients with little to no TR during mitral valve surgery. (IRB-HSR #18704: Evaluating the Benefit of Concurrent Tricuspid Valve Repair During Mitral Surgery)
- Neuroprotection Risk Reduction During AVR
Also through the CTSN, UVA is now enrolling patients in a trial to minimize risk of stroke and neurocognitive dysfunction during aortic valve surgery using novel cannulas. These devices are designed to capture any debris before embolization to the brain. Patients undergoing AVR (minimally invasive or open) may be eligible for this exciting study. (IRB-HSR #17894: Neuroprotection in Patients Undergoing Aortic Valve Replacement)
- TAVR for Low-Risk Patients
TAVR is currently FDA approved for high-risk patients (defined as a risk of mortality of > 15 percent) and is expected to be approved soon for intermediate-risk patients (defined as a risk of mortality of > 4 percent) based on results from the PARTNER 2 study, in which UVA was a leading center. “UVA performed TAVR in intermediate-risk patients with great success in a recent national study, with a zero percent mortality rate and zero percent stroke rate,“ says Ailawadi. UVA is now working to expand the pool of eligible patients even further with the PARTNER 3 trial beginning June 2016, which looks at low risk patients (defined as risk of mortality < 4 percent). Study participants will be randomized to receive either TAVR or surgery at our site. (IRB-HSR #18854: Placement of Aortic Transcatheter Valves)
- TMVR
The next frontier with new technologies is transcatheter mitral valve replacement (TMVR). UVA has been asked to participate in two revolutionary new trials (one of these is PRELUDE, IRB #18884) evaluating the feasibility and efficacy of valve replacement without opening the heart. These clinical trials of new experimental devices are expected to start later this year.
See more valve studies now enrolling at UVA.
To learn more about the treatments available at UVA for heart valve disease, schedule a visit from a UVA Advanced Cardiac Valve Center team member. Contact our Referral Services team for more information.