More than five million people in the U.S. are living with heart failure, and the rate of new diagnoses continues to rise. That’s why the UVA Heart and Vascular Center team is committed to discovering new ways to treat heart failure more effectively.
“UVA has been at forefront of clinical research that advances our knowledge of how to better treat patients with all forms of heart disease,” says cardiologist Scott Lim, MD, medical director of the UVA Advanced Cardiac Valve Center. “We’ve also been fortunate to have significant infrastructure to help us care for these patients by offering them the latest generation of new technology designed to help them better manage their heart disease.”
Reshaping the Heart
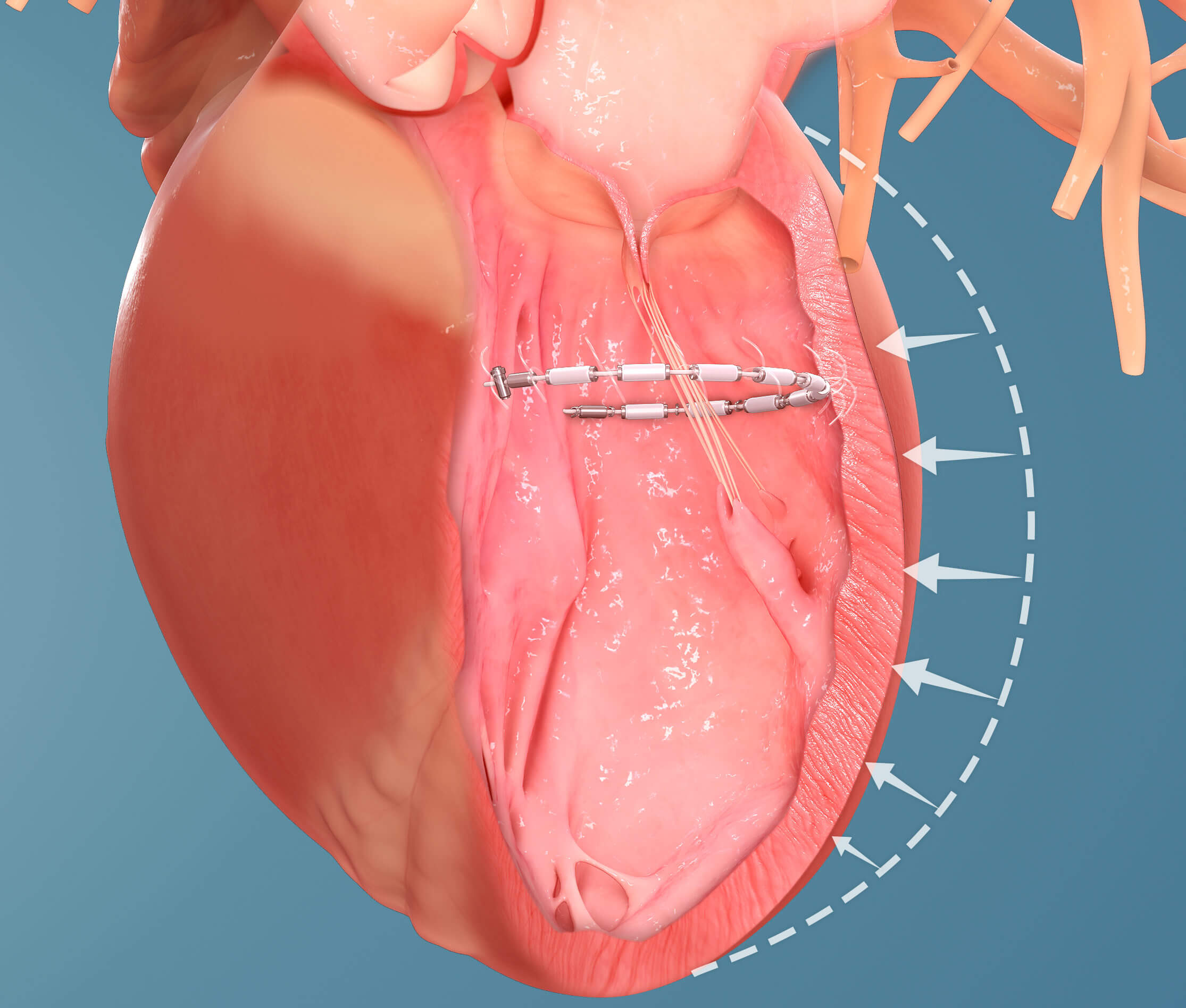
One such technology currently being investigated at UVA as part of an early feasibility study is the AccuCinch® Ventricular Repair System. This is one of the first technologies to be studied that seeks to reshape the heart to make it pump more effectively.
“In patients with a weak heart muscle, the heart compensates by getting bigger and more dilated, but this is a less efficient shape and the heart’s pumping capacity is diminished,” says Lim. “Putting a band inside the heart allows it to assume its proper shape and regain strength.”
How It Works
UVA recently completed its first minimally invasive AccuCinch transcatheter procedure. The device was threaded via a catheter through a vessel in the patient’s leg up to the left ventricle of the heart. It was then anchored into the heart muscle and tightened to cinch the heart muscle into the desired shape.
“Our team was quite pleased with how smoothly the procedure went,” says Lim. “The patient was awake and talking afterward. He stayed one night in the hospital and was able to go home the next day.”
Now Enrolling
UVA plans to enroll 15 patients in the AccuCinch feasibility study. Each will be followed for five years post-procedure. To be eligible for enrollment, patients must meet the following inclusion criteria.
- Male or female ages 18 years or older
- Left ventricular ejection fraction between 20% and 40%
- Ambulatory and in compliance with current prescribed medical therapy for heart failure.
In general, patients with other significant comorbidities are not eligible for enrollment. View the complete list of exclusion criteria at clinicaltrials.gov.
For more information, contact clinical research coordinator Linda Bailes.